Microsoft ends support for Internet Explorer on June 16, 2022.
We recommend using one of the browsers listed below.
- Microsoft Edge(Latest version)
- Mozilla Firefox(Latest version)
- Google Chrome(Latest version)
- Apple Safari(Latest version)
Please contact your browser provider for download and installation instructions.
July 30, 2025
NTT, Inc.
World's First Discovery of New Electronic State in Functional Oxides
― A lead for the development of future high-performance electronics materials ―
News Highlight
- We discovered that the electronic states of ruthenium and oxygen atoms in strontium ruthenium SrRuO3, which exhibits metallic and magnetic properties and is expected to be used in various electronics applications, actually differ, although they were previously thought to be strongly hybridized and integrated.
- This discovery clearly shows that the electron correlation of oxygen atoms is important in determining the physical properties of materials, which has been overlooked in conventional theories and material designs that consider only the electron-electron interaction (electron correlation) in transition-metals.
- These results provide new design guidelines for functional oxide materials and are expected to contribute to next-generation electronics using low-power magnetic memory and quantum devices.
![Conduction electrons in each electron orbital in the functional oxide SrRuO3: The interaction between electrons (electron correlation) in the Ru 4d-O 2p hybrid orbital of SrRuO3 was found to depend on the electron orbital. The Ru 4d orbitals (red cloverleaf-shaped orbitals) have weak electron correlation, and the electrons in these orbitals (red) contribute mainly to electrical conduction (red dashed arrows). On the other hand, in the O 2p orbitals (blue dumbbell-shaped orbitals), electrons (blue) are localized in the O 2p orbitals due to strong electron correlation, and they hardly contribute to electrical conduction [electrons move between sites by electron-electron scattering (blue dashed arrow)].](img/250730aa.png) Conduction electrons in each electron orbital in the functional oxide SrRuO3:
Conduction electrons in each electron orbital in the functional oxide SrRuO3:
The interaction between electrons (electron correlation) in the Ru 4d-O 2p hybrid orbital of SrRuO3 was found to depend on the electron orbital. The Ru 4d orbitals (red cloverleaf-shaped orbitals) have weak electron correlation, and the electrons in these orbitals (red) contribute mainly to electrical conduction (red dashed arrows). On the other hand, in the O 2p orbitals (blue dumbbell-shaped orbitals), electrons (blue) are localized in the O 2p orbitals due to strong electron correlation, and they hardly contribute to electrical conduction [electrons move between sites by electron-electron scattering (blue dashed arrow)].
Overview
A research group consisting of graduate students Yuichi Seki, (second-year master's at the time of the research), Kohdai Inagaki (second-year master's student at the time of the research), and Takahito Takeda (third-year Ph.D. student at the time of the research), along with Professor Masaaki Tanaka and Associate Professor Masaki Kobayashi (at the time of the research), from the Graduate School of Engineering, The University of Tokyo, and NTT, Inc. (Headquarters: Chiyoda, Tokyo; President and CEO: Akira Shimada; hereinafter, "NTT"), in collaboration with Emeritus Professor Atsushi Fujimori of the Graduate School of Science, The University of Tokyo, Yukiharu Takeda, Senior Principal Researcher at the Japan Atomic Energy Agency, and Group Leader Shin-ichi Fujimori, have examined the electronic states of strontium ruthenium SrRuO3 [Compound consisting of Sr (strontium), Ru (ruthenium), and O (oxygen)], a ferromagnetic metal, using photoemission spectroscopy2 with synchrotron radiation1. It was revealed for the first time in the world that the electron orbital of the oxygen atom has a different electronic state from that of the cation Ru due to strong electron correlation3. Functional oxides made from cationic transition-metal atoms and anionic oxygen atoms are a treasure trove of physical properties such as magnetism, dielectric properties, and superconductivity, and have been vigorously researched as next-generation electronics materials from both basic and applied perspectives. The physical properties of functional oxides are determined by the behavior of electrons (electronic states) at Fermi energy4. It has been generally considered that the electron orbitals of transition-metals and oxygen are strongly hybridized and have similar electronic states. This finding represents a result that could not have been predicted by conventional understanding or standard models of oxide research. It provides important insights for the future design of functional oxides and devices utilizing them.
This research will be published online in the U.S. scientific journal Physical Review Letters on July 25, 2025] (Eastern Standard Time).
Announcement
[Background of the study]
Functional oxides, which are compounds of transition-metal atoms and oxygen atoms, have been intensively studied as functional materials because they exhibit various physical properties such as superconductivity, giant magnetoresistance, and multiferroics5, which are expected to be used in future electronics, in addition to the electrical conductivity, magnetism, and dielectric properties of metals, semiconductors, and insulators used in conventional electronics. The complex motion of electrons caused by electrostatic repulsive force (Coulomb interaction) between electrons in materials is called "electron correlation," and electron correlation in transition-metals, which are cations, has been considered to play an important role in the expression of these physical properties. Therefore, few studies have focused on the electronic states of oxygen atoms. In addition, it has been difficult to produce high-quality functional oxides that are necessary for the precise examination of the electronic states of oxygen atoms. In recent years, in addition to electron correlations in transition-metals, which are cations in functional oxides, electron correlations in oxygen, which is an anion, have been discussed theoretically and experimentally. However, it is not clear how the electron correlation of oxygen affects the properties of functional oxides.
The functional oxide SrRuO3 has important properties for electronics applications such as metallic conduction, ferromagnetism, and perpendicular magnetic anisotropy, and has been studied for more than 60 years since its discovery. In particular, in recent years, it has been demonstrated that electronic states reflecting topological properties6, called magnetic Weyl semimetal states7, exist in ultrahigh-quality thin films, and they are attracting attention as materials with potential applications in topological spintronics. SrRuO3 forms an electronic state in which the electron orbitals8 of the cation Ru and the anion oxygen are hybridized, but it has not been known how the electronic state at the Fermi energy, which is responsible for the above characteristics, contributes to the physical properties of materials.
[Outline of the experiment]
In this study, in order to precisely examine the electronic properties of cation Ru and anion oxygen in SrRuO3, we performed photoemission spectroscopy using synchrotron radiation on SrRuO3 thin film, which has the highest crystallinity in the world, and precisely examined the electronic states at Fermi energy. By adjusting the energy of incident X-rays, this photoemission spectroscopy enables us to experimentally separate and examine the electronic states (partial density of states)9 derived from each electron orbital.
[Experimental results]
In this study, we found that the partial density of states derived from the electron orbital of the cation Ru crosses the Fermi energy and is metallic (electrons are itinerant; contribution to electrical conduction is large), while the partial density of states derived from the electron orbital of oxygen is almost zero at the Fermi energy and is close to an insulator (electrons are localized; contribution to electrical conduction is small) (Figure 1). Conventionally, it has been thought that the electron orbitals of Ru and oxygen in SrRuO3 are strongly hybridized and have the same shape independent of the electron orbitals (Figure 1). However, the observation results in this study differ from this conventional understanding. To investigate this, we experimentally estimated the magnitude of the electron correlation in the electron orbitals of oxygen atoms, and found that the electron correlation of oxygen atoms is several times larger than that of Ru atoms, which has been estimated from previous studies (Figure 2). Due to the strong electron correlation, the electronic state derived from the electron orbital of oxygen is close to that of an insulator, and contributes little to electrical conduction. This is the first experimental observation in the world to clearly demonstrate the influence of the electron correlation of oxygen on the electronic state at the Fermi energy, providing a new perspective in the long-standing field of functional oxides.
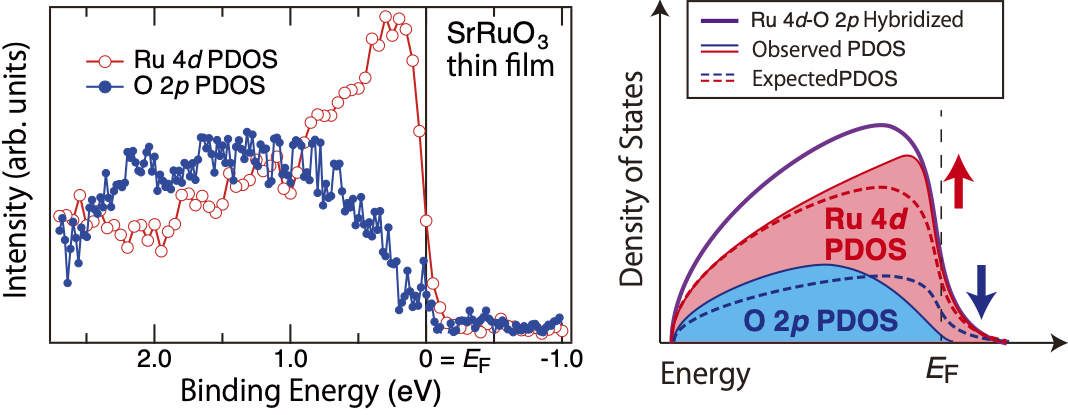 Figure 1 Electronic State of Functional Oxide SrRuO3
Figure 1 Electronic State of Functional Oxide SrRuO3
Note. (a) Partial density of states (PDOS) derived from Ru 4d electron orbitals (red) and O 2p electron orbitals (blue) observed by photoemission spectroscopy. (b) Schematic diagram of the Ru 4d-O 2p hybridized state. As indicated by the dashed line, Ru 4d and O 2p PDOS were thought to have the same shape although they were different in size. The solid lines show the experimental PDOS of each orbital (red and blue) and their sum, the Ru 4d-O 2p hybridized state (purple). Contrary to the predictions of existing theories, the partial densities of states derived from Ru electron orbitals, Ru 4d PDOS and O 2p PDOS, have different shapes. Ru 4d PDOS (red) has a high density of states at the Fermi energy EF, indicating metallic behavior, while O 2p PDOS (blue) has almost zero density of states at EF, indicating a state is close to that of an insulator. The arrows represent how each PDOS differs from theoretical predictions (Ru 4d PDOS is larger than expected, while the O 2p PDOS is smaller than expected).
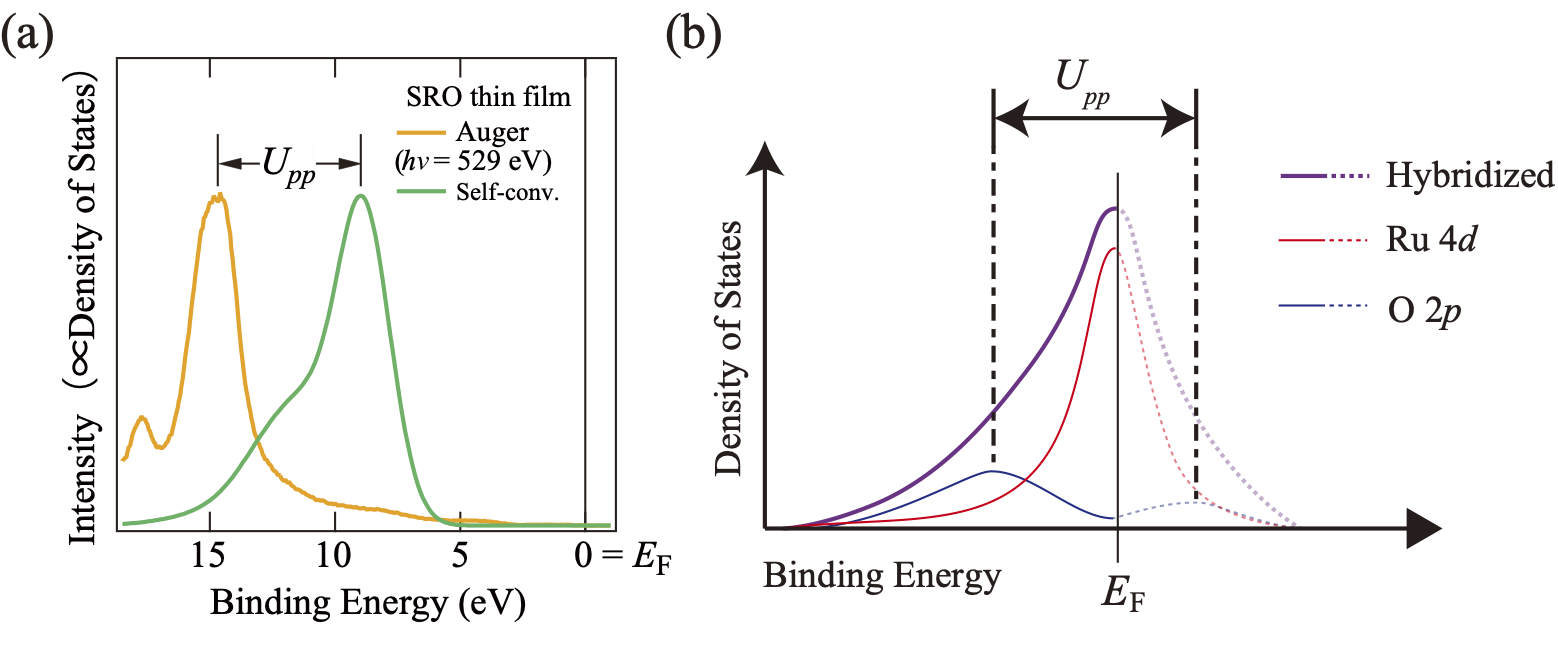 Figure 2 Electron Correlation of Oxygen in SrRuO3 (Upp)
Figure 2 Electron Correlation of Oxygen in SrRuO3 (Upp)
Note. (a) Estimation of electron correlation. Comparison between the Auger spectrum of oxygen (Orange, including electron correlation) and the self-convolution spectrum of the valence band (Green, no electron correlation). The peak energy difference reflects the magnitude of the electron correlation (Upp). The self-convolution spectrum is a spectrum obtained by converting the O 2p electronic state, which does not contribute to valence band hybridization, into a spectrum in the case of no electron correlation by self-convolution. (b) Schematic diagram of the Ru 4d-O 2p hybridized electronic state considering Upp. Due to the strong electron correlation of oxygen, the density of states (DOS) at the EF of O 2p PDOS (blue) decreases to nearly zero, which is close to an insulator state.
[Technical Points]
(1) Synchrotron radiation photoelectron spectroscopy
At synchrotron radiation facilities, where high-brightness X-rays can be used, research on materials using cutting-edge spectroscopy is progressing day by day. Synchrotron radiation is characterized by its high brightness and the ability to change the energy of X-rays. In photoemission spectroscopy using synchrotron radiation, by matching the X-ray energy to the absorption energy of the electron orbital of a specific element, it is possible to increase the signal intensity of the partial density of states derived from a specific electron orbital and observe it. In this research, we succeeded in observing the partial densities of states of the Ru 4d and O 2p electron orbitals using X-rays corresponding to their absorption energies.
(2) High quality thin film fabrication technology
SrRuO3 thin films with a crystal structure called perovskite structure were fabricated by the oxide molecular beam epitaxy method (ML-MBE) using machine learning developed by NTT in 2019. In order to fabricate high-quality thin films, precise adjustment of thin film fabrication conditions is essential, and has been done by trial and error by researchers and engineers. The ML-MBE method uses a statistical machine learning method called Bayesian optimization to efficiently optimize the thin film fabrication conditions. This technology has made it possible to fabricate ultrahigh-quality SrRuO3 thin films (Figure 3), in which Sr, Ru, and O are ordered at the atomic level.
[Roles of each parties]
In this research, the University of Tokyo and NTT jointly developed an experimental plan and performed photoemission spectroscopy measurements using synchrotron radiation at SPring-8, a large-scale synchrotron radiation facility. NTT fabricated high-quality SrRuO3 thin films by the oxide ML-MBE method and evaluated the physical properties of the thin films. The University of Tokyo performed photoemission spectroscopy measurements and data analysis on SrRuO3 thin films provided by NTT.
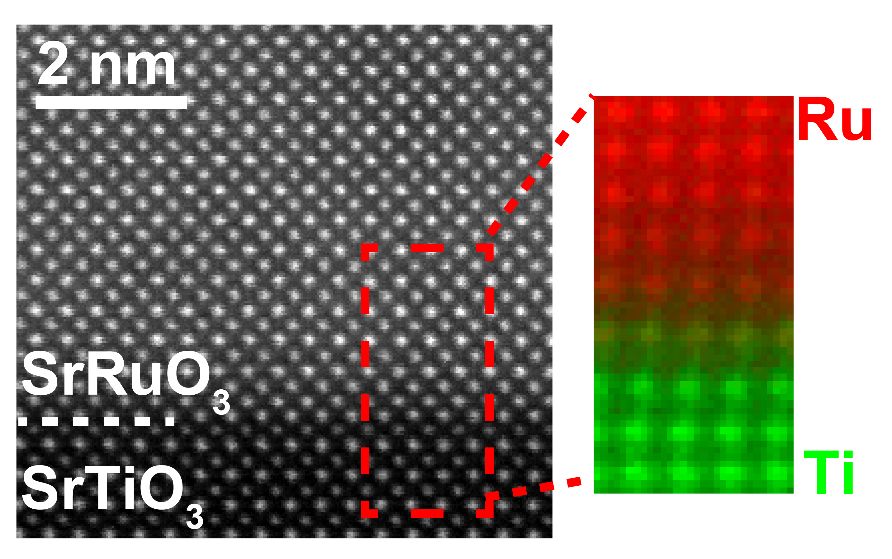 Figure 3 Electron Microscopy Image of SrRuO3 Thin Film
Figure 3 Electron Microscopy Image of SrRuO3 Thin Film
(Source: Press Release "First observation of quantum transport phenomena peculiar to an exotic state" https://group.ntt/en/newsrelease/2020/10/09/201009a.html)
[Future Development]
In this study, we discovered that the electronic states of transition-metals and oxygen atoms, which were thought to be strongly hybridized and integrated, are different in functional oxide SrRuO3. It has been generally considered that the electron correlation of transition-metals, which are cations, has an important effect on the physical properties of functional oxides. This result, which overturns the conventional theory, indicates the need to re-evaluate the influence of electron correlation of oxygen in functional oxides. It is a new concept that can be widely applied to other materials in which electron orbitals of transition-metals and oxygen are hybridized.
In the future, incorporating oxygen electron correlation into theoretical frameworks is expected to enhance the accuracy of materials design. Through the use of theoretical and large-scale computational predictions of physical properties, it is anticipated that this will lead to the functional design and simulation of functional oxides, ultimately enabling the development of magnetic memory and quantum devices based on new principles.
Information on presenters and researchers
The University of Tokyo
Department of Electrical Engineering and Information Systems, Graduate School of Engineering
Masaki Kobayashi (Associate Professor at the time of the research)
Also affiliated with the Center for Spintronics Research Network (CSRN), Graduate School of Engineering, The University of Tokyo
Currently: Senior Researcher, NTT Basic Research Laboratories
Yuichi Seki (Master's student at the time of the research)
Kohdai Inagaki (Master's student at the time of the research)
Takahito Takeda (Ph.D. student at the time of the research)
Currently: Assistant Professor, Graduate School of Engineering, The University of Tokyo
Masaaki Tanaka, Professor
Also affiliated with the Center for Spintronics Research Network (CSRN), Graduate School of Engineering, The University of Tokyo
Graduate School of Science
Atsushi Fujimori, Emeritus Professor
Also affiliated with: Visiting Professor, Center for Quantum Technology, National Tsing Hua University, Taiwan
NTT Basic Research Laboratories
Yuki K. Wakabayashi, Distinguished Researcher
Yoshitaka Taniyasu, Senior Distinguished Researcher / Group Leader
Hideki Yamamoto, Senior Distinguished Researcher
Yoshiharu Krockenberger, Senior Researcher
Japan Atomic Energy Agency (JAEA), Nuclear Science Research Institute,
Materials Science Research Center
Yukiharu Takeda, Senior Principal Researcher
Shin-ichi Fujimori, Group Leader
Paper Information
Journal Name: Physical Review Letters
Title: Correlated Ligand Electrons in the Transition-Metal Oxide SrRuO3
Author: Yuichi Seki, Yuki K. Wakabayashi, Takahito Takeda, Kohdai Inagaki, Shin-ichi Fujimori, Yukiharu Takeda, Atsushi Fujimori, Yoshitaka Taniyasu, Hideki Yamamoto, Yoshiharu Krockenberger, Masaaki Tanaka, and Masaki Kobayashi*
DOI: 10.1103/n9qh-6739
URL: https://journals.aps.org/prl/abstract/10.1103/n9qh-6739
Research Grant
This research was supported by the following programs and institutions: the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Challenging Research (Exploratory) [19K21961], Grant-in-Aid for Scientific Research (S) [20H05650 and 22H04948], and Grant-in-Aid for Scientific Research (C) [22K03535]; the Japan Science and Technology Agency (JST) CREST program [JPMJCR1777]; the Spintronics Research Network of Japan (Spin-RNJ); the National Science and Technology Council (NSTC) of Taiwan under the Special Research Project [NSTC 113-2112-M-007-033]; the Nanotechnology Platform Program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan [JPMXP1222AE0031]; the JAEA Advanced Characterization Platform [2022A-E23]; and the Proposal Review Committee of JASRI [2020A3841].
Glossary
1. Synchrotron radiation
Synchrotron radiation refers to light such as ultraviolet and X-rays that is emitted tangentially when electrons, accelerated to extremely high speeds in a ring-shaped ultra-high vacuum chamber, are deflected by magnetic fields. Because this light is intense and broadband (white light), specific wavelengths can be selectively extracted for use. This enables highly sensitive spectroscopic measurements and detailed analysis of material properties. For this reason, synchrotron radiation is considered essential for advancing materials development. In Japan, synchrotron radiation facilities such as SPring-8 (in Harima) and the Photon Factory (in Tsukuba) are widely utilized.
2. Photoemission Spectroscopy
When short-wavelength (high-energy) X-rays are directed at a material, electrons are emitted from its surface due to the photoelectric effect. These emitted electrons are called "photoelectrons." Because photoelectrons carry information from inside the material, analyzing them makes it possible to examine the electronic states that determine the material's properties. With synchrotron radiation, the energy of the X-rays can be tuned. By matching the X-ray energy to that of a specific electron orbital, the electronic states originating from that orbital (partial density of states) can be selectively observed.
3. Electron correlation
Electrons interact with each other through repulsive electrostatic forces, known as Coulomb interactions. Even within a material, electrons experience mutual repulsion, and the interactions among electrons in a many-electron system are referred to as "electron correlation." Electron correlation is particularly pronounced in spatially localized orbitals, such as d or f orbitals. It is known to play a key role in the emergence of quantum phenomena such as superconductivity and metal-insulator transitions.
4. Fermi energy
Electrons in a material fill the available energy levels starting from the lowest-energy orbitals of the constituent elements (ions). The energy level of the highest occupied state at absolute zero temperature is called the Fermi energy. The properties of a material are determined by the electronic states near the Fermi energy. In metals, there are electronic states (density of states) at the Fermi energy, whereas in insulators, no electronic states exist at the Fermi energy.
5. Multiferroics
In materials, ferroic orders include ferromagnetism, ferroelectricity, and ferroelasticity. These refer to states in which magnetization, electric polarization, or strain spontaneously arises under thermal equilibrium without an external field. When multiple ferroic orders coexist within a material due to the breaking of various symmetries, and these orders are strongly coupled to one another, such materials are referred to as multiferroics. In multiferroic materials, strong coupling between magnetic and electric properties can lead to large magnetoelectric effects, such as magnetic-field-induced reversal of electric polarization.
6. Topological properties
Topology is a concept in mathematics, specifically in the field of topology (also called topological geometry), which classifies and understands objects based on universal properties that remain unchanged under continuous deformations. A common example compares a coffee cup with a handle (which has one hole between the cup and the handle) and a doughnut with a hole in the center. Since both have one hole and can be continuously deformed into each other, they are considered equivalent from a topological perspective.
7. Magnetic Weyl semimetal state
In solid-state materials, there are two types of Weyl semimetal states. One is the non-magnetic Weyl semimetal state, which occurs independently of magnetism. The other is the magnetic Weyl semimetal state, where the intrinsic magnetism of the material plays an essential role in its emergence. The former was discovered in 2015 by multiple research groups in the non-magnetic compound TaAs, composed of tantalum and arsenic. The latter was discovered two years later, in 2017, in Mn3Sn, composed of manganese and tin. The magnetic Weyl semimetal state demonstrated in SrRuO3 belongs to this latter category.
The behavior of electrons in the Weyl semimetal state is understood as the behavior of quasiparticles called Weyl fermions. These particles are massless and were predicted in 1929 by the German physicist Hermann Weyl. Although extensively studied in high-energy particle physics, their existence had not been confirmed in particle physics experiments. Recently, it has become clear that such particles can exist in exotic states realized within solid materials known as Weyl semimetal states. This discovery has led to active research in condensed matter physics.
Topological properties refer to characteristics of states realized in materials that can be well understood using the concepts of topology. The magnetic Weyl semimetal state includes such topological properties.
8. Electron Orbitals
In atoms that make up materials, quantum mechanical effects cause electrons to occupy discrete energy levels called electron orbitals. These orbitals are known as the K shell (s orbitals), L shell (p orbitals), and M shell (d orbitals). Each orbital can accommodate a fixed number of electrons. The outermost electron orbitals of the constituent elements hybridize, forming valence bands with continuous energy levels rather than discrete ones.
9. Partial density of states
The density of states is a physical quantity that represents how many electrons can occupy a small energy range within the continuous energy distribution of the valence band. It is possible to define the density of states for each electron orbital that forms the valence band, and the density of states originating from a specific orbital is called the "partial density of states." The total density of states of a material corresponds to the sum of all partial densities of states that make up the valence band.
In photoelectron spectroscopy using synchrotron radiation, a technique called resonant photoelectron spectroscopy, which utilizes core-level excitation, allows experimental observation of the partial density of states originating from specific electron orbitals.
About NTT
NTT contributes to a sustainable society through the power of innovation. We are a leading global technology company providing services to consumers and businesses as a mobile operator, infrastructure, networks, applications, and consulting provider. Our offerings include digital business consulting, managed application services, workplace and cloud solutions, data center and edge computing, all supported by our deep global industry expertise. We are over $90B in revenue and 340,000 employees, with $3B in annual R&D investments. Our operations span across 80+ countries and regions, allowing us to serve clients in over 190 of them. We serve over 75% of Fortune Global 100 companies, thousands of other enterprise and government clients and millions of consumers.
Media Contacts
(For details of the research, please contact the presenter.)
NTT Basic Research Laboratories
Masaki Kobayashi, Senior Researcher
NTT Science and Core Technology Laboratory Group
Public Relations
Inquiry form
Information is current as of the date of issue of the individual press release.
Please be advised that information may be outdated after that point.
NTT STORY
WEB media that thinks about the future with NTT